

Higher and foundation tiers
When you think about chemical reactions you need to consider two key words or terms which are often used in describing chemical reactions; that is the system and the surroundings. The system is the chemicals or reacting molecules while the surroundings are the test-tubes or beakers in which the chemical reactions happen. The surroundings also include the classroom and everything else around it. Basically everything except the reacting chemicals are the surroundings.
Some common chemical reactions that you will have likely carried out in the lab are:
Can you guess what all these reactions have in common? Well all these chemical reactions release energy to the surroundings. They are all exothermic reactions.
An exothermic reaction releases energy to the surroundings, usually in the form of heat. A thermometer (part of the surroundings) would measure an increase in temperature during an exothermic reaction.
The neutralisation reaction between an acid and an alkali is a good example of an exothermic reaction that takes place in solution. Even using fairly dilute solutions a thermometer placed in a beaker containing a mixture of say hydrochloric acid and the alkali sodium hydroxide; as shown below; will record a temperature rise of around 25°C. Many oxidation reactions such as the combustion of metals such as magnesium are also highly exothermic reactions and will release large amounts of heat and light energy to the surroundings.

The reacting chemicals (the system) in a chemical reaction act as a store of chemical energy. That energy is stored in the chemical bonds holding the atoms together in the molecules. When an exothermic reaction takes place the system loses energy to the surroundings, mainly as heat. As that energy moves out of the system the temperature of the surroundings increases and the reaction mixture feels warm. Remember you are part of the surroundings; so if you hold a test tube containing a chemical reaction and it feels warm then the system; the reacting chemicals is transferring heat energy to your hand; which is part of the surroundings.

The law of conservation of energy states that energy cannot be created or destroyed. It can only be transferred from one place to another 🔄.
So in a chemical reaction if the system loses energy the surroundings must gain exactly the same amount.
Energy never disappears, it just moves or transfers from one type to another. 🔥
Now think about the law of conservation of energy. Energy cannot be created or destroyed; it can only be transferred from one place to another. So if the reacting chemicals lose energy the surroundings must gain exactly the same amount. In an exothermic reaction the product molecules end up with less energy than the reactant molecules. The difference in energy stored between them is the amount of energy transferred to the surroundings. That is what it means to say energy is conserved in a chemical reaction. We can show this transfer of energy in both exothermic and endothermic reactions using an energy profile diagram.
Examples of everyday exothermic reactions are shown in the two images below; which show a self-heating coffee can and a hand warmer in use.

Have you ever sucked on a sherbet sweet and felt your mouth getting slightly cooler? This happens because there is an endothermic reaction taking place in your mouth. Sour sherbet sweets contain citric acid and a base called sodium bicarbonate (sodium hydrogen carbonate). In your mouth these chemicals react to produce carbon dioxide gas, which gives the fizzing sensation. The reaction between citric acid and sodium bicarbonate is endothermic. It removes energy from the surroundings and in this case the surroundings are your mouth. That is why you feel a cooling sensation as you enjoy eating fizzy sherbet sweets.
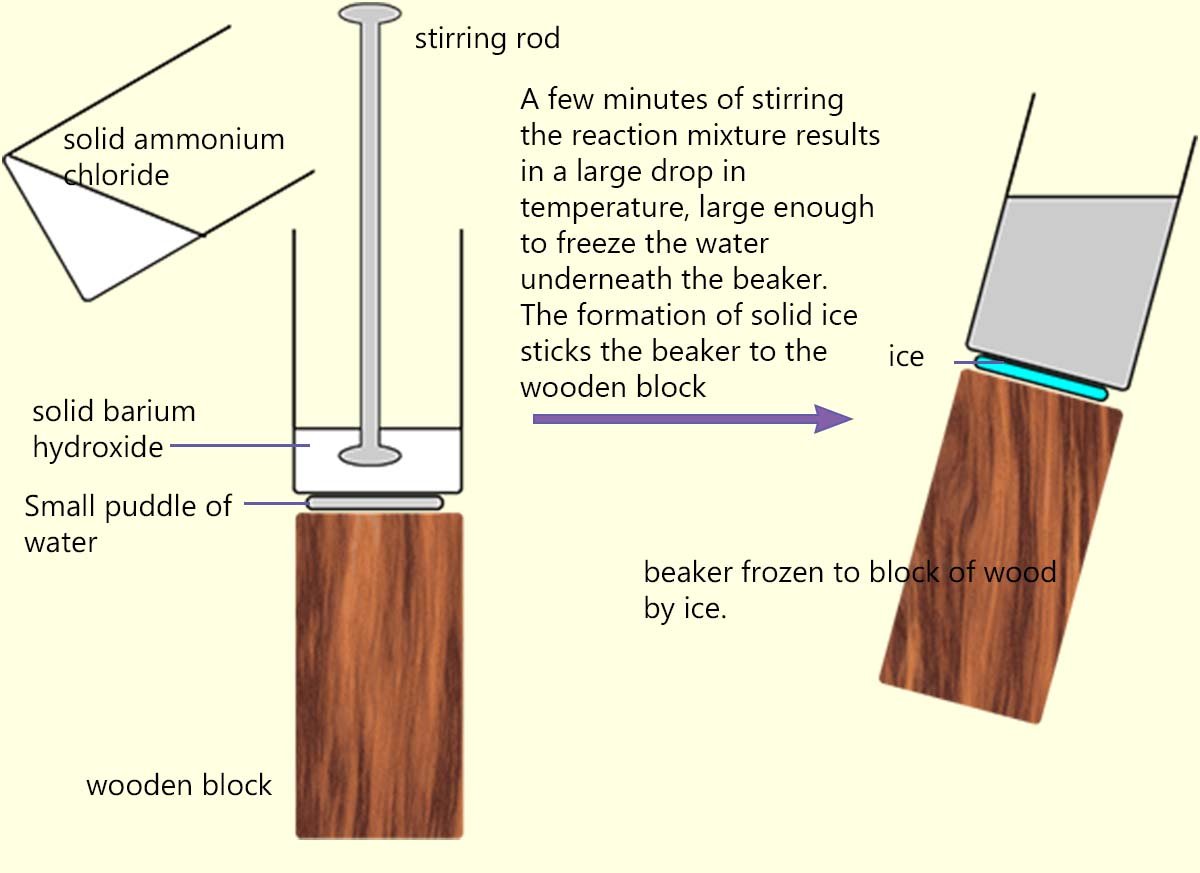
Endothermic reactions are much less common than exothermic reactions.
One of the most spectacular endothermic
reactions that you may see is where solid ammonium chloride is added to a beaker containing solid barium hydroxide.
The beaker containing the solid barium hydroxide is sitting on top of a block of wood on which a small pool
of water has been added as shown in the image opposite. When the two solids are added together and stirred vigorously so much
heat is removed
from the surroundings by the reacting chemicals that the pool of water under the beaker freezes and forms a solid block of ice. This ice
is so thick that it will stick the beaker to the wooden block.
The temperature changes are also very dramatic here.
If the experiment is done in a lab at room temperature (25°C) then during the experiment a thermometer placed in the reacting chemicals may read as low -10°C; a temperature drop of 35°C. In this experiment the reacting chemicals;
the system; has gained
energy from the surroundings. As a consequence the
temperature of the surroundings, which includes the thermometer' has dropped since they have lost heat energy.
An endothermic reaction removes heat energy from the surroundings. A thermometer (part of the surroundings) would measure a decrease in temperature during an endothermic reaction.

Many athletes can easily pick up an injury such as a muscle tear or strain to their joints, this will result in soft tissue damage occurring and the injury site may begin to swell. Applying a cold sports injury pack to the injury site can result in a decrease in fluid build up and swelling at the site of the injury. These sports injury packs rely on an
endothermic reaction to apply instant cooling to an injury to help reduce swelling and pain. Inside most instant cold packs there are chemicals such as ammonium nitrate or urea. When the pack is squeezed, the solid dissolves in water and this dissolving process is endothermic, meaning it absorbs energy from the surroundings and causes the pack to become cold. The
endothermic reactions that occur in sports injury cooling packs can also be applied to your forehead to relieve migraines and headaches.
Other types of sports injury pack contain chemicals that when activated react exothermically to release heat into the site of injury or pain. This type of sports injury pack is often used to treat long term injuries. The
heat released causes the blood vessels to dilate or widen which helps deliver more blood into the injury site and this can helps in the healing of damaged tissues.
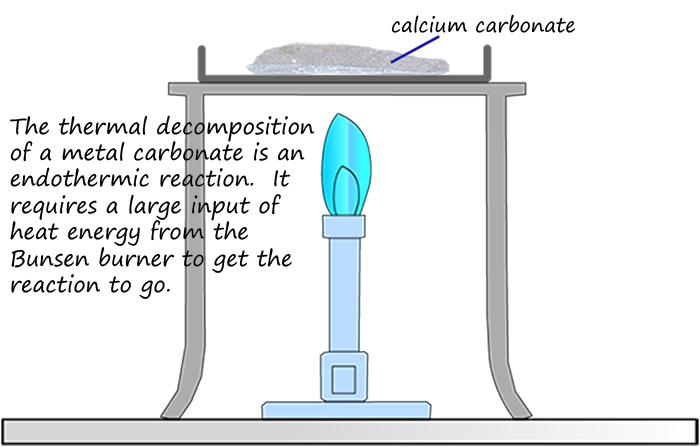
Decomposition reactions occur when a substance breaks down into smaller simpler substances. Metal carbonates for example will decompose or break up when heated strongly to form a metal oxide and release the gas carbon dioxide; as shown in the image opposite. However a large amount of heat energy is required before this decomposition reaction will take place; so the thermal decomposition of a metal carbonate is another example of an endothermic reaction, an equation for the thermal decomposition of calcium carbonate is shown below:
Photosynthesis is an endothermic reaction because it requires an input of energy to take place. However this energy is not heat, instead plants absorb light energy from the Sun using chlorophyll in their leaves. That light energy is transferred into the chemical bonds of glucose. In other words the products of photosynthesis contain more stored energy than the reactants. This shows that an endothermic reaction does not have to absorb heat. It simply means that energy is taken in from the surroundings, in photosynthesis that energy source is light not heat.

Try the quick quiz below to review your knowledge on exothermic and endothermic reactions.
Why not use the summary table below to make up a few flash cards on exothermic and endothermic reactions and their common features?
| Feature | Exothermic 🔥 | Endothermic ❄️ |
|---|---|---|
| Energy transfer | Releases energy to the surroundings | Removes energy from the surroundings |
| Temperature change | Temperature of surroundings increases | Temperature of surroundings decreases |
| Energy in products | Products contain less energy than reactants | Products contain more energy than reactants |
| Energy profile diagram | Products lower than reactants 📉 | Products higher than reactants 📈 |
| Examples | Combustion, neutralisation, respiration | Sherbet reaction, cold packs, ammonium chloride + barium hydroxide |
| Misconception 🤔 | Correct Understanding ✅ |
|---|---|
| Endothermic reactions always absorb heat. | Endothermic reactions absorb energy. The energy source could be light (as in photosynthesis) and not heat. |
| If a reaction feels cold it must be exothermic. | If it feels cold it is usually endothermic ❄️ because energy is being removed from the surroundings. |
| Exothermic reactions create energy. | Energy cannot be created. It is transferred from the system to the surroundings 🔄. |
| Products in an exothermic reaction contain more energy. | In an exothermic reaction the products contain less energy than the reactants. The difference is transferred to the surroundings. |